BENEFITS
High Accuracy
The UT + Diagnostics Covid-19 Antigen Detection Kit delivers high-precision results when evaluated with clinical specimen, using commercial RT-PCR kit as the reference method.(1)
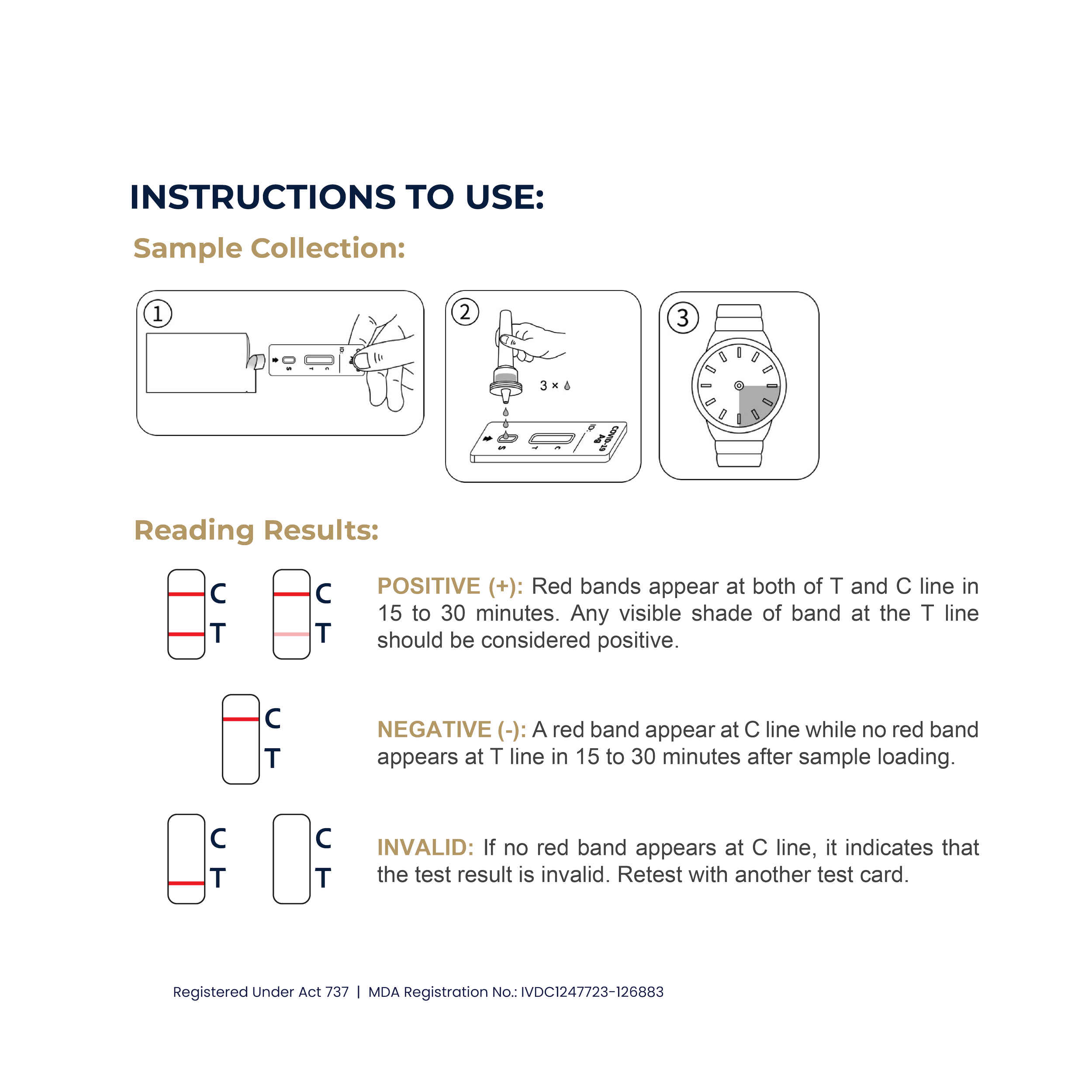
Easy, Clear, Rapid Result
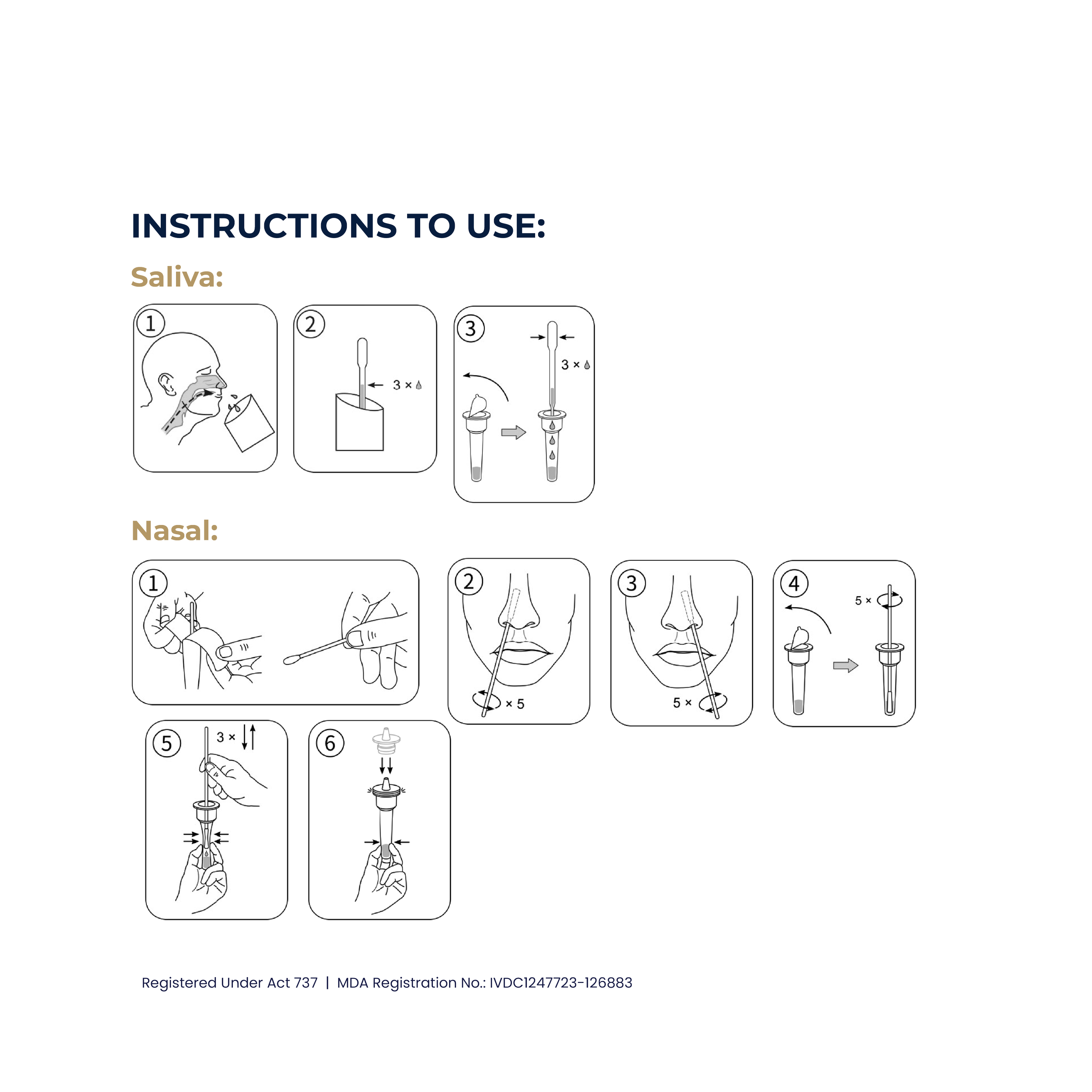
The UT + Diagnostics Covid-19 Antigen Detection Kit detects the presence or absence of SARS-CoV-2 antigen in 15 minutes. No equipment is required to process the specimens or read the results.
Non-Invasive
The UT + Diagnostics Covid-19 Antigen Detection Kit use saliva or nasal swab which are non-invasive and less uncomfortable than traditional nasopharyngeal swabs, making them more acceptable to individuals, especially children and those with sensitivities.
Bulk Testing
The UT + Diagnostics Covid-19 Antigen Detection Kit are scalable and suitable for bulk testing, making it easier to screen large populations for COVID-19.
Cost-Effective
The UT + Diagnostics Covid-19 Antigen Detection Kit are more cost-effective than traditional polymerase chain reaction PCR tests, which require specialized equipment and trained personnel.
PERFORMANCE
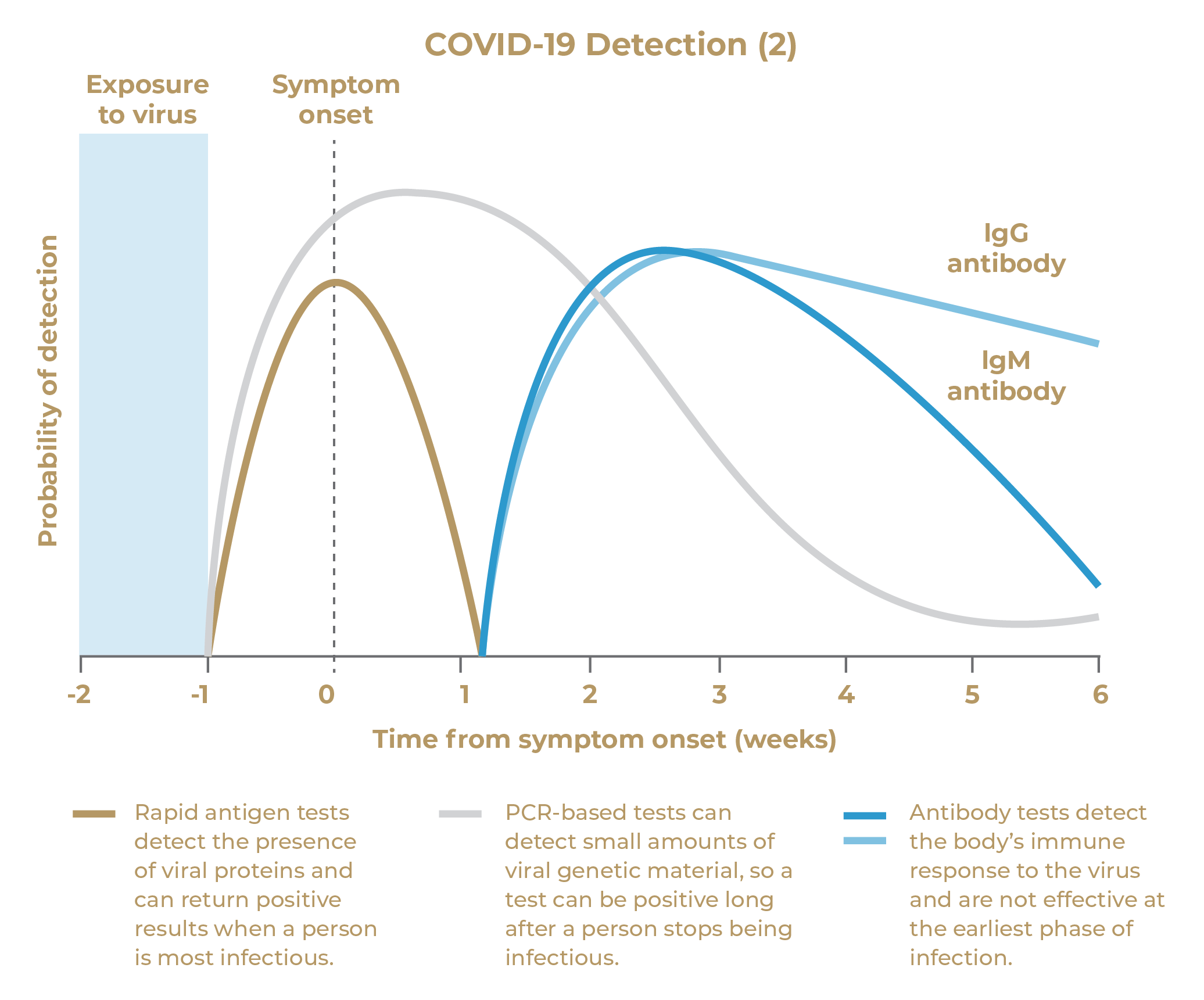
▲ The UT + Diagnostics Covid-19 Antigen Detection Kit is sensitive enough to detect patients with a high viral load during early onset of symptoms. This includes, for example, pre-symptomatic and early symptomatic cases or low RT-PCR cycle threshold cases, which likely account for a significant number of transmissions.(3)
SPECIFICATIONS
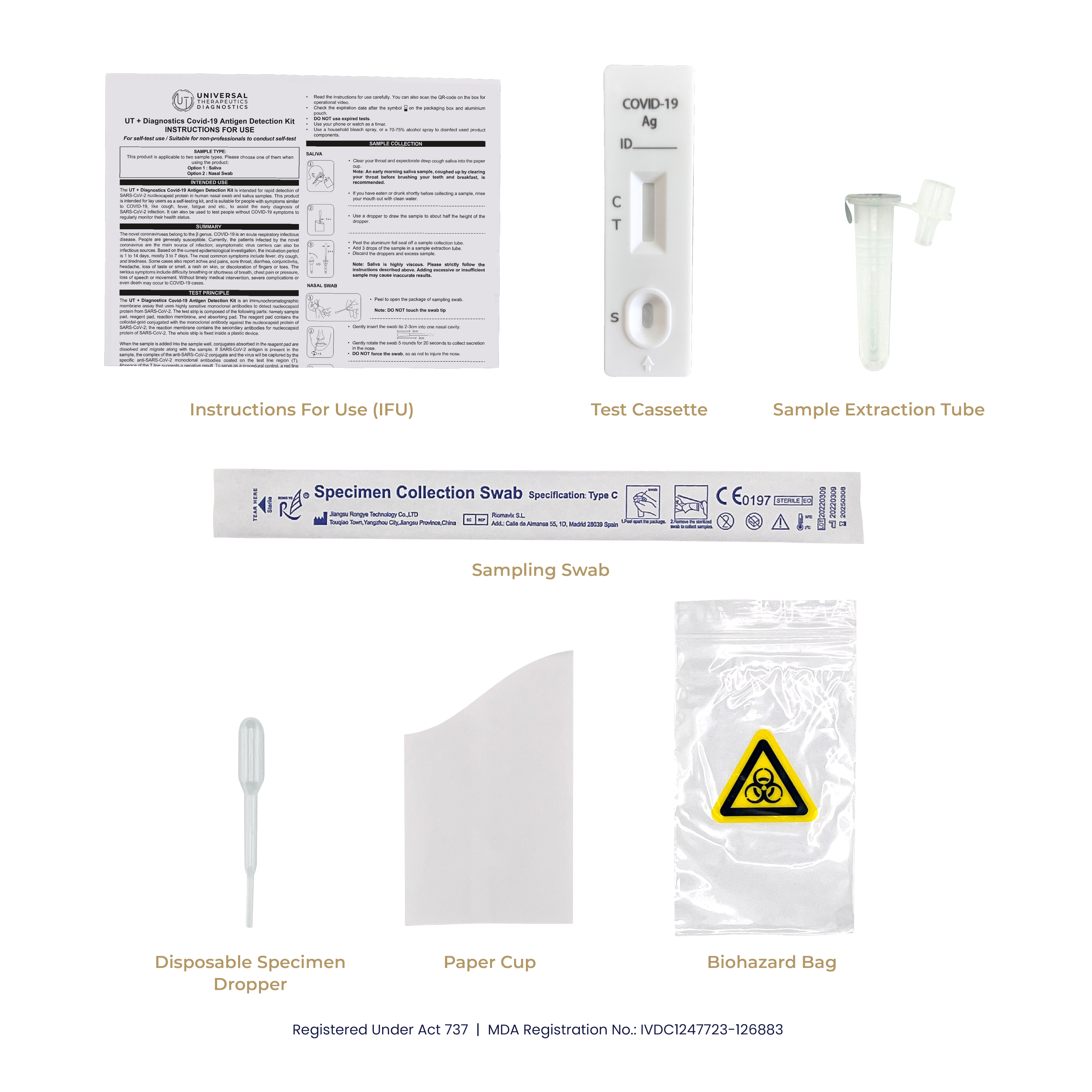
CONTENTS
1 test device in foil pouch
1 paper cup
1 dropper
1 sampling swab
1 sample extraction tube and tube cap
1 biohazard bag
1 package insert (instructions for use)
REFERENCES
1. Universal Therapeutics Sdn Bhd. (2023). Instructions for Use: The UT + Diagnostics Covid-19 Antigen Detection Kit.
2. Guglielmi G. (2020). Fast coronavirus tests: what they can and can't do. Nature 585, 496-498. doi: https://doi.org/10.1038/d41586-020-02661-2.
3. European Centre for Disease Prevention and Control (2020). Options for the use of rapid antigen tests for COVID-19 in the EU/EEA and the UK. ECDC Stockholm. https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19_0.pdf.
-
You may submit your test results via https://mysejahtera.moh.gov.my/index.php?option=com_sppagebuilder&view=page&id=17