OCTOBER 2023 REGULATORY OVERVIEW
BY MEDTECH CONSULTING- A SUBSIDIARY OF UNIVERSAL THERAPEUTICS
MALAYSIA
MDA to Cease Issuance of Certificate of Free Sale (CFS) for Export Only Medical Devices
23rd October 2023
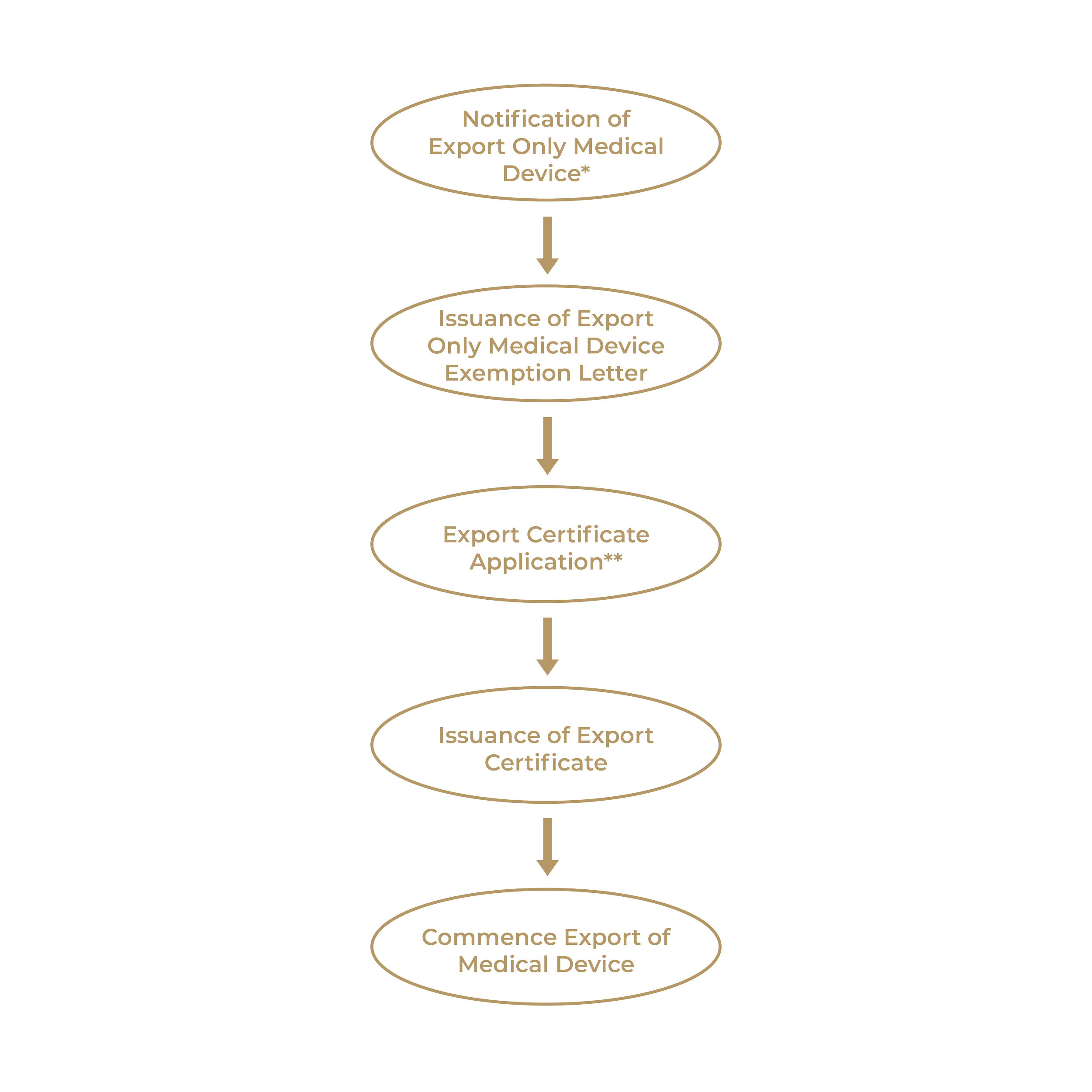
Effective 1st October 2023, the Medical Device Authority (MDA) will only issue Certificate of Free Sale (CFS) for medical devices that have undergone registration in Malaysia. Unregistered medical devices intended solely for export will now be subjected to the issuance of an Export Certificate.
CFS certificates issued prior to the effective date will remain valid until their respective expiration date.
It should be noted that the CFS and Export Certificate are NOT a mandatory requirement under the Act 737. Instead, it is designed to facilitate the export of medical devices from Malaysia.
The process of applying for an Export Certificate for Export Only Medical Devices is depicted below:
The original MDA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
Footnotes:
*Notification of Export Only Medical Device: https://www.mda.gov.my/industry/notification-for-export-only-medical-device.html
**Export Certificate Application Process: https://www.mda.gov.my/industry/export-certificate.html
MDA Advances Complete Implementation of Electronic Medical Device Registration Certificate to 1st November 2023
26th October 2023
The Medical Device Authority (MDA) has officially accelerated the timeline for the complete implementation of electronic medical device registration certificates. Previously scheduled for 1st January 2024, this pivotal transition will now come into effect on 1st November 2023. Following this change, the issuance of physical certificates for approved medical device registration applications will cease for those submitted after the 1st November deadline.
Electronic medical device registration certificates can be downloaded from MeDC@St 2.0, an online system designed for application of licenses and certificates with the MDA.
The original MDA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
SINGAPORE
Latest Revision of the Medical Devices Product Classification Guide
2nd October 2023
The Health Sciences Authority (HSA) in Singapore published the second revision of the GL-06 Medical Device Product Classification Guide on 2nd October 2023.
Notable changes within the updated guidelines include:
- Eye irrigation solution (primary mode of action is physical: lubricating or physical washing) is classified as a Medical Device. For example, eye irrigation solutions for relief of irritated eyes (due to particles such as dust or smoke) via physical washing of eye surface.
- Eye drops (primary mode of action via osmosis) and emergency eye washes are classified as Non-Medical Device.
- Emergency skin washes intended solely for washing of chemical spills / splash are classified as Non-Medical Device.
The latest Medical Devices Product Classification Guide (Rev 2, October 2023) is accessible here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
Consultation on GN-20 Guidance on Clinical Evaluation
27th October 2023
A preliminary version of the medical device guidance document, GN-20 Guidance on Clinical Evaluation, is released by the Health Sciences Authority (HSA), Singapore for the purpose of soliciting input from stakeholders. The consultation period is scheduled to take place from 27th October to 30th November 2023. A copy of the draft document is available for reference here.
All stakeholders are invited to review the draft and document their feedback in the form provided here. The completed forms can be sent to HSA_MD_INFO@hsa.gov.sg with the subject of the email clearly marked as “Feedback on GN-20”.
The original HSA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.