FEBRUARY 2024 REGULATORY OVERVIEW
Monthly regulatory review by MedTech Consulting- a subsidiary of Universal Therapeutics.
MALAYSIA
Publication of the Medical Device (Compounding of Offences) Regulations 2024.
16th February 2024
The Medical Device Authority (MDA) would like to announce that the Medical Device Regulations (Compounding of Offenses) 2024 have been gazetted and came into effect starting 30th January 2024.
MDA informs that any establishment or individual must comply with the existing laws currently in force. The implementation of these regulations is in line with the powers granted under Section 71 and 79 of the Medical Device Act 2012 [Act 737].
The original MDA announcement can be accessed here, while the Medical Device (Compounding of Offences) Regulations 2024 can be downloaded here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form here.
SINGAPORE
There are no relevant announcements from HSA in February.
JANUARY 2024 REGULATORY OVERVIEW
Monthly regulatory review by Medtech Consulting- a subsidiary of Universal Therapeutics.
MALAYSIA
First Edition Guidance Document on Importation of Medical Device for Personal Use
30th January 2024
The guidance document - MDA/GD/0066 - was published by the Medical Device Authority (MDA) to guide the import of medical devices for the purpose of personal use.
A medical device for “personal use” is defined in the guidance document as “a medical device which is brought into Malaysia for the use of a particular individual only and not to be placed in the market”. The person who imports medical device for personal use is exempted from the requirement of an establishment license under Section 15 of Act 737.
The following are requirements set forth in the guidance document for import or purchase (including via online platform) of medical devices for personal use:
a) only for home-use medical devices;
b) the medical device is for own use or the use of immediate family;
c) all labels and labelling information that comes with the medical device shall be retained;
d) quantity for medical device shall be appropriate according to the type of the medical device;
e) no same or similar medical device(s) is/are registered in Malaysia; and
f) to obtain a formal prescription or letter of recommendation on the medical device(s) from a registered healthcare professional upon request from the Authority.
The original MDA announcement can be accessed here, while the guidance document MDA/GD/0066 can be downloaded here.
For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
First Edition Guidance Document on Medical Device Post Market Information Exchange for ASEAN Member States
30th January 2024
Following the recent Twelfth Meeting of the ASEAN Medical Device Committee (AMDC) held at Brunei Darussalam in September 2023, the Medical Device Authority (MDA) has decided to adopt the Guideline on Medical Device Post Market Information Exchange for ASEAN Member States that was ratified during the meeting.
The first edition of this guidance document, namely the Guidance Document on Medical Device Post Market Information Exchange for ASEAN Member States (MDA/GD/067), was published on 30th January 2024 and serves to provide guidance on the exchange of post market information of medical devices among ASEAN Member States (AMS). This post market information may include events leading or highly likely to lead to unanticipated public health threat, as well as observations from national trend analysis.
The original MDA announcement can be accessed here, while the guidance document MDA/GD/0067 can be downloaded here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
SINGAPORE
Regulatory Fee Revision for Medical Devices and other Health Products
8th January 2024
The Health Sciences Authority (HSA) has announced an increase in fees related to the cost of registration, licensing, notification and permit issuance for medical devices and for other health products. This fee revision will take effect starting on 1st July 2024, and is necessary to recover part of the costs for the services rendered to businesses.
The Health Sciences Authority (HSA), Singapore has officially announced a forthcoming adjustment in fees pertaining to the registration, licensing, notification, and permit issuance for medical devices and other health products. This revised fee structure is slated to come into effect from 1st July 2024.
The rationale behind this fee revision is to recuperate a portion of the expenses associated with the services provided to businesses in the respective sectors. The HSA emphasises the necessity of this measure to ensure sustainable support for the delivery of quality services in the realm of healthcare product regulation.
The original HSA announcement can be accessed here, while the list of revised fees for Medical Devices can be downloaded here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
DECEMBER 2023 REGULATORY OVERVIEW
BY MEDTECH CONSULTING- A SUBSIDIARY OF UNIVERSAL THERAPEUTICS
MALAYSIA
Updates to Guidance Documents on Harmonised Medical Device Classification and Borderline Products in ASEAN
12th December 2023
The Medical Device Authority (MDA) has released updated editions of two Guidance Documents, namely the Harmonised Classification of Medical Devices in ASEAN (MDA/GD/0062) and Harmonised Borderline Products in ASEAN (MDA/GD/0063). These revisions are in accordance with the latest decisions of the ASEAN Medical Device Committee (AMDC).
MDA/GD/0062 Harmonised Classification of Medical Devices
The second and latest edition includes additional medical device classification categories, commencing from the "Cardiovascular Devices" category on page 12 through page 19 of the guidance document.
MDA/GD/0063 Harmonised Borderline Products in ASEAN
Twenty-four new products have been incorporated into the list of borderline medical devices with medicine, spanning from item no. 181 to 204.
The original MDA announcements can be accessed
here and here. For further enquiries, please contact us at our contact form here.
SINGAPORE
None for the month of December.
Clarification Statement- UT + Diagnostics Covid-19 Antigen Detection Kit (2-in-1 Saliva/Nasal Swab)
Dear Valued Distributors,
UT + Diagnostics Covid-19 Antigen Detection Kit (2-in-1 Saliva/Nasal Swab)
1. Universal Therapeutics wishes to clarify that our proprietary devices branded under “Universal Therapeutics Diagnostics” is proudly manufactured and assembled in Malaysia (Made in Malaysia).
Kuala Lumpur, Malaysia, 1 December 2023-
Dear Valued Distributors,
UT + Diagnostics Covid-19 Antigen Detection Kit (2-in-1 Saliva/Nasal Swab)
Universal Therapeutics wishes to clarify that our proprietary devices branded under “Universal Therapeutics Diagnostics” is proudly manufactured and assembled in Malaysia (Made in Malaysia).
We are issuing this statement with reference to “UT + Diagnostics Covid-19 Antigen Detection Kit (2-in-1 Saliva/Nasal Swab)”. This device is not manufactured or associated with “Newgene Bioengineering”.
“UT + Diagnostics Covid-19 Antigen Detection Kit (2-in-1 Saliva/Nasal Swab)”
This device registered with Medical Device Authority (MDA) with the registration no.: IVDC1247723-126883.
This device is proudly manufactured and assembled in Malaysia.
To verify the authenticity of the registration, visit: https://mdar.mda.gov.my/frontend/web/index.php?r=carian%2Fview&id=76211
For sales or further clarifications, please contact us at: enquiries@universal-therapeutics.com.
Thank you for your continued support.
NOVEMBER 2023 REGULATORY OVERVIEW
Monthly Regulatory Review by Medtech Consulting- a subsidiary of Universal Therapeutics.
BY MEDTECH CONSULTING- A SUBSIDIARY OF UNIVERSAL THERAPEUTICS
MALAYSIA
Updates to Medical Device Advertisement Application Process
8th November 2023
All medical device advertisement applications to the Medical Device Authority (MDA) shall be submitted via email to advertisement@mda.gov.my starting 1st December 2023. Hard copy application forms will no longer be processed after this date.
The application form (Annex A) can be downloaded here.
The original MDA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form here.
Footnotes:
*Notification of Export Only Medical Device: https://www.mda.gov.my/industry/notification-for-export-only-medical-device.html
**Export Certificate Application Process: https://www.mda.gov.my/industry/export-certificate.html
Approaches to Register IVD Analysers
16th November 2023
The Medical Device Authority (MDA) recently published an infographic that describes several options for registration of IVD Analysers in Malaysia.
Outlined below are the three distinct approaches highlighted by the MDA within the infographic:
1. IVD analysers that are intended specifically by the manufacturer to be used in in vitro diagnostic procedures are classified as Class A. Reagents and kits are classified in their own right.
Examples:
· Enzyme immunoassay analysers,
· PCR thermocycler,
· Microplate reader,
· Clinical chemistry analyser,
· Instrument for automated purification of nucleic acids and automated nucleic acid extractor
2. For analysers registered in conjunction with reagents, calibrators, controls, buffer/washing solutions, product classification should be guided by the Class of the associated reagent.
Examples:
· Clinical chemistry analyser + Reagent assay + Diluent,
· Immunoassay analyser-coagulation + D-dimer reagent + D-dimer control
3. Analysers with independent measuring function, without the need for additional reagents, is classified according to the intended purpose of the analysis (including instrument controls or instrument quality control).
Example:
· Cell counting analyser used in haematology,
· Ion-selective electrodes,
· Instruments measuring blood gases or glucose via its sensors,
· Specific gravity measurements in urine analysis,
· Mass spectrometer for bacteria identification,
· Erythrocyte sedimentation rate analyser
The original MDA announcement, inclusive of the infographic, is accessible here.
For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form here.
SINGAPORE
None for the month of November.
OCTOBER 2023 REGULATORY OVERVIEW
Monthly Regulatory Review by Medtech Consulting- a subsidiary of Universal Therapeutics.
BY MEDTECH CONSULTING- A SUBSIDIARY OF UNIVERSAL THERAPEUTICS
MALAYSIA
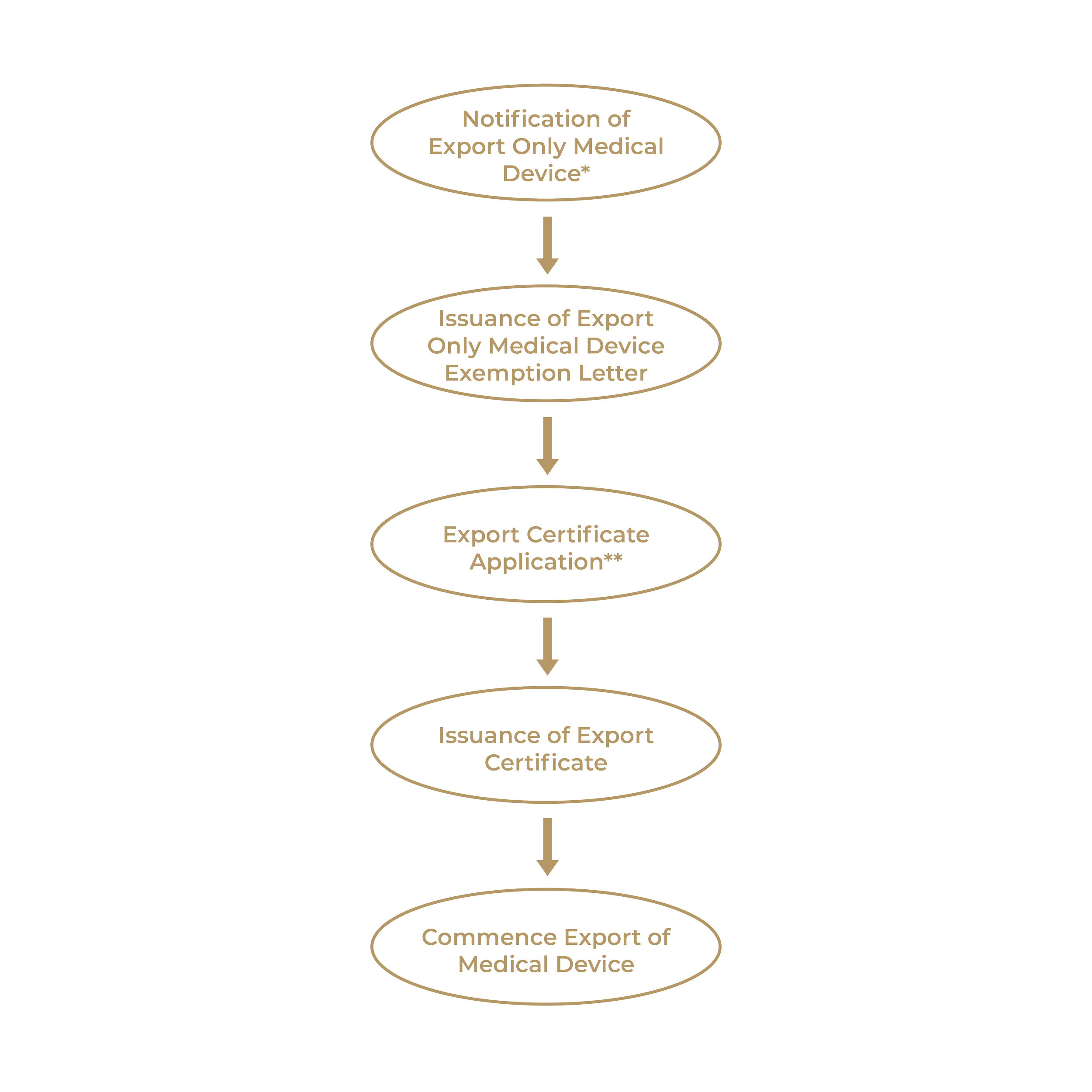
MDA to Cease Issuance of Certificate of Free Sale (CFS) for Export Only Medical Devices
23rd October 2023
Effective 1st October 2023, the Medical Device Authority (MDA) will only issue Certificate of Free Sale (CFS) for medical devices that have undergone registration in Malaysia. Unregistered medical devices intended solely for export will now be subjected to the issuance of an Export Certificate.
CFS certificates issued prior to the effective date will remain valid until their respective expiration date.
It should be noted that the CFS and Export Certificate are NOT a mandatory requirement under the Act 737. Instead, it is designed to facilitate the export of medical devices from Malaysia.
The process of applying for an Export Certificate for Export Only Medical Devices is depicted below:
The original MDA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
Footnotes:
*Notification of Export Only Medical Device: https://www.mda.gov.my/industry/notification-for-export-only-medical-device.html
**Export Certificate Application Process: https://www.mda.gov.my/industry/export-certificate.html
MDA Advances Complete Implementation of Electronic Medical Device Registration Certificate to 1st November 2023
26th October 2023
The Medical Device Authority (MDA) has officially accelerated the timeline for the complete implementation of electronic medical device registration certificates. Previously scheduled for 1st January 2024, this pivotal transition will now come into effect on 1st November 2023. Following this change, the issuance of physical certificates for approved medical device registration applications will cease for those submitted after the 1st November deadline.
Electronic medical device registration certificates can be downloaded from MeDC@St 2.0, an online system designed for application of licenses and certificates with the MDA.
The original MDA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
SINGAPORE
Latest Revision of the Medical Devices Product Classification Guide
2nd October 2023
The Health Sciences Authority (HSA) in Singapore published the second revision of the GL-06 Medical Device Product Classification Guide on 2nd October 2023.
Notable changes within the updated guidelines include:
- Eye irrigation solution (primary mode of action is physical: lubricating or physical washing) is classified as a Medical Device. For example, eye irrigation solutions for relief of irritated eyes (due to particles such as dust or smoke) via physical washing of eye surface.
- Eye drops (primary mode of action via osmosis) and emergency eye washes are classified as Non-Medical Device.
- Emergency skin washes intended solely for washing of chemical spills / splash are classified as Non-Medical Device.
The latest Medical Devices Product Classification Guide (Rev 2, October 2023) is accessible here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
Consultation on GN-20 Guidance on Clinical Evaluation
27th October 2023
A preliminary version of the medical device guidance document, GN-20 Guidance on Clinical Evaluation, is released by the Health Sciences Authority (HSA), Singapore for the purpose of soliciting input from stakeholders. The consultation period is scheduled to take place from 27th October to 30th November 2023. A copy of the draft document is available for reference here.
All stakeholders are invited to review the draft and document their feedback in the form provided here. The completed forms can be sent to HSA_MD_INFO@hsa.gov.sg with the subject of the email clearly marked as “Feedback on GN-20”.
The original HSA announcement can be accessed here. For further enquiries, please contact us at Mtresponder@medtechconsulting.com.my or utilise our contact form.
Universal Therapeutics Diagnostics and Community Healthcare Clinic (CHC Clinic) Collaborate to Provide High-Accuracy AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid)
Kuala Lumpur, Malaysia, 17 October 2023- Universal Therapeutics Diagnostics is excited to announce its strategic partnership with Community Healthcare Clinic, a trusted leader in HIV and STD screening services. This collaboration introduces the highly accurate and reliable AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid), clinically assessed to have an accuracy of 99.61%, a sensitivity of 99.23%, and a specificity of 100%.
Kuala Lumpur, Malaysia, 17 October 2023- Universal Therapeutics Diagnostics and Community Healthcare Clinic (CHC Clinic) collaborate to offer high-accuracy AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid).
Universal Therapeutics Diagnostics is excited to announce its strategic partnership with Community Healthcare Clinic, a trusted leader in HIV and STD screening services. This collaboration introduces the highly accurate and reliable AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid), clinically assessed to have an accuracy of 99.61%, a sensitivity of 99.23%, and a specificity of 100%.
Community Healthcare Clinic, renowned for its commitment to patients and quality healthcare, recognises the potential of the AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) as a superior HIV 1/2 testing solution that efficiently meets patient needs. This user-friendly, non-invasive kit provides a sophisticated alternative for individuals seeking an HIV test without the inconvenience of traditional methods.
Universal Therapeutics Diagnostics remains steadfast in its commitment to continuously improve performance and clinical data. Through this collaboration, the real-time collection of accuracy data during patient testing will further elevate the quality of diagnostics. Dato' Sri Samuel Chai, Chief Executive Officer of Universal Therapeutics, underscores the importance of this partnership, stating, "Our collaboration with Community Healthcare Clinic empowers us to enhance diagnostics with real-time insights, ensuring the delivery of reliable, accurate and trusted test kits to our patients."
Mr Ramesh Vadiveloo, the Community Healthcare Manager of Community Healthcare Clinic, expresses his satisfaction with this innovative alternative, noting, "The AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) provides patients with a non-traditional testing method that aligns perfectly with their preferences."
This collaboration between Universal Therapeutics Diagnostics and Community Healthcare Clinic marks a significant step forward in offering patient-centric, accurate, and convenient HIV testing solutions.
For more information:
Universal Therapeutics Diagnostics Sdn Bhd
Cassandra YAP (Miss)
Corporate Communications Executive
Email: PR@universal-therapeutics.com
Office: +60 3 9213 0318
Community Healthcare Clinic (CHC Clinic)
Ramesh Vadiveloo (he/him)
Clinic Manager
2, Level 1, Jalan Haji Salleh, Sentul 51100 Kuala Lumpur
Clinic Tel: 0340513611
Clinic WhatsApp: 01121434572
About Universal Therapeutics Diagnostics
Universal Therapeutics Diagnostics Sdn Bhd is a manufacturer of In Vitro Diagnostics (IVDs) and Rapid Test Kits. We are committed to excellence and innovation, delivering reliable, safe and effective medical solutions to meet the evolving needs of the healthcare industry. With ISO13485:2016 QMS certification and a GDPMD license, the company provides a comprehensive suite of diagnostic solutions, including OEM and ODM services. The company's product range encompasses vital diagnostic tests such as HIV (Oral Fluid), Dengue, Malaria, Ovulation, Pregnancy, Drugs of Abuse (DOA), Syphilis, Hepatitis C, and Influenza A&B tests. All our kits are made with pride in Malaysia.
For more information, visit www.universal-therapeutics.com
About Community Healthcare Clinic (CHC Clinic)
CHC Clinic is a community-based clinic working with key affected populations on HIV and AIDS, gender identity and sexual health. CHC Clinic offers a full range of services for all aspects of health, including testing, consultation, counselling, treatment and prevention, all under one roof. A lack of information has led to a lot of discrimination when it comes to STI’s, especially HIV. At CHCC we make that information available, without taboo or shame.
For more information, visit www.chcclinic.org
Universal Therapeutics Diagnostics and The Red Clinic Collaborate to Provide High-Accuracy AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid)
Kuala Lumpur, Malaysia, 15 September 2023 - Universal Therapeutics Diagnostics proudly announces its strategic partnership with The Red Clinic, a respected leader in HIV Screening. This collaboration introduces the cutting-edge AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) with an impressive accuracy of 99.61%, sensitivity of 99.23%, and specificity of 100%.
Dato’ Sri Samuel Chai and Dr. Andrew Yap
Kuala Lumpur, Malaysia, 15 September 2023 - Universal Therapeutics Diagnostics proudly announces its strategic partnership with The Red Clinic, a respected leader in HIV Screening. This collaboration introduces the cutting-edge AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) with an impressive accuracy of 99.61%, sensitivity of 99.23%, and specificity of 100%.
The Red Clinic, known for its reliability and patient-centred approach, recognises the potential of the AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) as an alternate HIV 1/2 testing solution that meets patient needs efficiently. This non-invasive and convenient kit offers an advanced option for those seeking an alternative to traditional testing methods.
Universal Therapeutics Diagnostics believes in continuously enhancing its performance and clinical data. Through this collaboration, real-time accuracy data collection during patient diagnosis will further enhance the quality of diagnostics. Dato' Sri Samuel Chai, Chief Executive Officer of Universal Therapeutics, emphasises the significance of the partnership, stating, "Our alliance with The Red Clinic enables us to elevate our diagnostics with real-time insights, ensuring the delivery of high-quality test kits to our patients."
Dr. Andrew Yap, founder of The Red Clinic, expresses his satisfaction with this innovative alternative, stating, "The AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid) offers patients a non-traditional testing method that aligns with their preferences."
This collaboration between Universal Therapeutics Diagnostics and The Red Clinic represents a significant stride towards offering patient-centric, accurate, and convenient HIV testing solutions.
For more information:
Universal Therapeutics Diagnostics Sdn Bhd
(A Subsidary of Universal Therapeutics Group Sdn Bhd)
Cassandra YAP (Miss)
Corporate Communications Executive
Email: PR@universal-therapeutics.com
Office: +60 3 9213 0318
Web: www.universal-therapeutics.com
The Red Clinic
Dr Andrew YAP
Founder and Director
Email: andrew@theredclinic.com
Web: www.theredclinic.com
About Universal Therapeutics Diagnostics
UT Diagnostics Sdn Bhd is a manufacturer of In Vitro Diagnostics (IVDs) and Rapid Test Kits. We are committed to excellence and innovation, delivering reliable, safe and effective medical solutions to meet the evolving needs of the healthcare industry. With ISO13485:2016 QMS certification and a GDPMD license, the company provides a comprehensive suite of diagnostic solutions, including OEM and ODM services. The company's product range encompasses vital diagnostic tests such as HIV (Oral Fluid), Dengue, Malaria, Ovulation, Pregnancy, Drugs of Abuse (DOA), Syphilis, Hepatitis C, and Influenza A&B tests. All our kits are made with pride in Malaysia.
For more information, visit www.universal-therapeutics.com.
Comprehensive Care at The Red Clinic
Embark on your path to optimal health with The Red Clinic, a trusted general practice committed to healthcare excellence. With a long history of participating in HIV research, our dedicated team combines advanced medical care, personalized attention, and a holistic ethos. Equipped with modern facilities and patient-centricity, we provide a range of services including medical check-ups, mental health support, dietetics, medical sonography and precise HIV testing. We believe in creating community through healthcare.
For more information, please visit www.theredclinic.com.
Universal Therapeutics Diagnostics Launches “Que Sarah, Sarah”- 1 Step Pregnancy Test Cassette and Test Pen
Kuala Lumpur, Malaysia, 3 July 2023 – Universal Therapeutics Diagnostics, a leading medical device manufacturer based in Malaysia, is proud to announce the release of two pregnancy test kits. The Que Sarah, Sarah 1 Step Pregnancy Test Cassette and Que Sarah, Sarah 1 Step Pregnancy Test Pen have been meticulously designed to provide accurate and reliable results, offering women a convenient and effective means of early pregnancy detection in the comfort of their own homes.
Kuala Lumpur, Malaysia, 3 July 2023 – Universal Therapeutics Diagnostics, a leading medical device manufacturer based in Malaysia, is proud to announce the release of two pregnancy test kits. The Que Sarah, Sarah 1 Step Pregnancy Test Cassette and Que Sarah, Sarah 1 Step Pregnancy Test Pen have been meticulously designed to provide accurate and reliable results, offering women a convenient and effective means of early pregnancy detection in the comfort of their own homes.
Certified with the ISO13485:2016 Quality Management System (QMS), Universal Therapeutics Diagnostics upholds the highest standards of product quality and safety. This certification underscores the company's commitment to delivering exceptional healthcare solutions to women worldwide.
The Que Sarah, Sarah 1 Step Pregnancy Test Cassette is an intuitive and self-performing immunoassay that enables the qualitative determination of human chorionic gonadotropin (HCG) hormone in urine. By leveraging advanced technology, this test kit offers early detection of pregnancy with exceptional accuracy. The cassette format ensures user-friendly handling and clear result interpretation, empowering women to monitor their reproductive health discreetly and confidently.
Complementing the cassette format, the Que Sarah, Sarah 1 Step Pregnancy Test Pen provides women with an alternative option for early pregnancy testing. Featuring a sleek and compact design, this pen-style test kit offers convenience and discretion. With a simple urine application, users can easily determine their pregnancy status through the pen's intuitive result display. The test pen is specifically designed to cater to the modern lifestyle, ensuring ease of use and reliability without compromising accuracy.
Both the Que Sarah, Sarah 1 Step Pregnancy Test Cassette and the Que Sarah, Sarah 1 Step Pregnancy Test Pen have obtained the necessary licensing from the Medical Device Authority (MDA) of the Ministry of Health Malaysia. This regulatory approval ensures that the products comply with stringent quality and safety requirements, providing users with utmost peace of mind when utilising these test kits.
As a Malaysian manufacturer, Universal Therapeutics Diagnostics takes great pride in the production of these pregnancy test kits, which are entirely Made in Malaysia. The company remains committed to contributing to the local healthcare industry and delivering world-class medical devices that address the evolving needs of women worldwide.
"We are excited to introduce the Que Sarah, Sarah 1 Step Pregnancy Test Cassette and Que Sarah, Sarah 1 Step Pregnancy Test Pen to the market," said Dato’ Sri Samuel Chai, Chief Executive Officer of Universal Therapeutics Diagnostics. "Our development team, led by Datin Sri Lynnette Yeo, Chief Strategist, has worked tirelessly to develop these innovative and user-friendly products, ensuring accuracy, reliability, and convenience for women seeking early pregnancy detection. With our ISO13485:2016 certification and manufacturer’s licence from the Medical Device Authority, we guarantee the highest quality standards and compliance with regulatory requirements."
The Que Sarah, Sarah 1 Step Pregnancy Test Cassette and Que Sarah, Sarah 1 Step Pregnancy Test Pen are now available for purchase through selected retailers and online platforms. Universal Therapeutics Diagnostics encourages women to take control of their reproductive health and experience the convenience and accuracy offered by these advanced pregnancy test kits.
For more information, visit: https://www.universal-therapeutics.com/products/p/ut-diagnostics-pregnancy-rapid-test-pen
For media inquiries, please contact:
Melissa, GOH (Miss)
Communications Manager
Universal Therapeutics Group Sdn Bhd
PR@universal-therapeutics.com
+60 3 9213 0318
About Universal Therapeutics Diagnostics:
A fully owned subsidiary of Universal Therapeutics Group, Universal Therapeutics Diagnostics is a leading medical device manufacturer based in Malaysia. The company specialises in developing innovative and reliable healthcare solutions, with a particular focus on women's health. Universal Therapeutics Diagnostics is committed to providing high-quality medical devices that empower individuals to manage their health effectively.
Universal Therapeutics Launches Groundbreaking AwarerPRO™ HIV 1/2 Rapid Test (Oral Fluid)
Kuala Lumpur, Malaysia, 19 June 2023 - Universal Therapeutics, a leading healthcare company dedicated to advancing medical solutions, is excited to announce the successful registration of the AwarerPRO™ 1/2 HIV Rapid Test Kit (Oral Fluid). This innovative test kit, with an impressive 99.61% accuracy rate independently verified by third parties, is poised to revolutionise HIV testing and significantly impact the healthcare industry.
Kuala Lumpur, Malaysia, 19 June 2023 - Universal Therapeutics, a leading healthcare company dedicated to advancing medical solutions, is excited to announce the successful registration of the AwarerPRO™ 1/2 HIV Rapid Test Kit (Oral Fluid). This innovative test kit, with an impressive 99.61% accuracy rate independently verified by third parties, is poised to revolutionise HIV testing and significantly impact the healthcare industry.
The AwarerPRO™ 1/2 HIV Rapid Test Kit introduces a breakthrough testing method utilising oral fluid, ensuring exceptional accuracy while offering a convenient and user-friendly experience. Bid farewell to messy procedures and discomfort; our kit is designed to simplify and streamline the testing process.
We proudly acknowledge the remarkable efforts and unwavering dedication of our team, who have consistently planned, developed, and conceptualized this remarkable medical device. Working tirelessly over the course of 18 months, they have ensured that every aspect of the AwarerPRO™ 1/2 HIV Rapid Test Kit adheres to the highest standards of quality and effectiveness.
As a company committed to continuous improvement, we remain dedicated to refining and enhancing this medical device. Our team will explore new possibilities, conduct further research, and collaborate with field experts to deliver the most advanced and reliable HIV testing solutions.
This achievement would not have been possible without the support and trust of our partners, healthcare professionals, and customers. We express our deepest gratitude for their unwavering belief in our mission and for providing us with the opportunity to contribute to global healthcare advancements.
We cordially invite everyone to join us in celebrating this significant milestone and to help spread the word about the AwarerPRO™ 1/2 HIV Rapid Test Kit. Together, we can make a substantial difference in the fight against HIV, improving lives and promoting early detection.
Announcement of Director Resignation
Kuala Lumpur, Malaysia, 20 February, 2023- The Management wishes to announce that Mr. Sidney Soares has ceased all functions of management and directorship at Universal Therapeutics Sdn Bhd and its subsidiaries from 20 February 2023. Universal Therapeutics thanks Mr. Soares for his dedicated service and contributions during his tenure and wishes him well in all his future endeavours. Moving forward, please direct all correspondence and queries intended for Mr. Soares to UTResponder@universal-therapeutics.com.
Kuala Lumpur, Malaysia, 20 February, 2023- The Management wishes to announce that Mr. Sidney Soares has ceased all functions of management and directorship at Universal Therapeutics Sdn Bhd and its subsidiaries from 20 February 2023.
Universal Therapeutics thanks Mr. Soares for his dedicated service and contributions during his tenure and wishes him well in all his future endeavours. Moving forward, please direct all correspondence and queries intended for Mr. Soares to UTResponder@universal-therapeutics.com.
We would like to take this opportunity to assure our stakeholders, partners, and clients that there has been a seamless transition of Mr. Soares’ duties and there will be no disruption to our services and offerings. Universal Therapeutics remains committed to pursuing its mission and vision with vigour.
We are grateful for all your continued support.
Bringing Joy to Local Community with Back to School Program
Kuala Lumpur, Malaysia - On January 7th, 2023, Universal Therapeutics and its subsidiary UT Pharmacy had the honor of partnering with Selangor State Assemblywoman YB Puan Michelle Ng to present 400 bursaries to young students as part of their Back to School program. The program was launched with the aim of fostering a love for education and promoting progress in the community.
Kuala Lumpur, Malaysia - On January 7th, 2023, Universal Therapeutics and its subsidiary UT Pharmacy had the honor of partnering with Selangor State Assemblywoman YB Puan Michelle Ng to present 400 bursaries to young students as part of their Back to School program. The program was launched with the aim of fostering a love for education and promoting progress in the community.
As the new school term starts, many families face the challenge of balancing school expenses with household needs. We understands the importance of education and does not want families to have to choose between school supplies and putting food on the table. That is why the Back to School program was created, to support families and ensure that every child has the opportunity to succeed.
Universal Therapeutics is grateful for the opportunity to support these students in their academic pursuits and is eager to see the positive impact this program will have on their future. The company is committed to making a difference in the community and will continue to support education in the future.
"Education is the foundation for progress and success, and we are proud to play a small role in shaping the future of these young students," said our CEO, Dato’ Sri Samuel Chai. "We believe that 'giving is a true blessing' and are excited to see the positive impact this program will have on our community."
For more information on this release and our community initiatives, please contact:
Cassandra Bowden (Miss)
External Communications Executive
Email: CAS@universal-therapeutics.com
Universal Therapeutics Diagnostics Sdn Bhd receives formal approval from Medical Device Authority (MDA), Malaysia our in-house manufacturing licence
Kuala Lumpur, Malaysia, 1 November 2022- Universal Therapeutics Diagnostics Sdn Bhd (“UTD”) receives the official nod from its competent and governing authority Medical Device Authority (MDA, Ministry of Health), Malaysia to commence her In- Vitro Diagnostics (“IVD”) production in its own facility.
Proudly “In-house” and “Made in Malaysia”, the plant boasts a menu of IVDs for medical practitioners and home users. UTD’s current focus will be pregnancy, infectious diseases, drug, dengue, and malaria IVDs.
For Immediate Release
1 November 2022
Kuala Lumpur, Malaysia, 1 November 2022- Universal Therapeutics Diagnostics Sdn Bhd (“UTD”) receives the official nod from its competent and governing authority Medical Device Authority (MDA, Ministry of Health), Malaysia to commence her In- Vitro Diagnostics (“IVD”) production in its own facility.
Proudly “In-house” and “Made in Malaysia”, the plant boasts a menu of IVDs for medical practitioners and home users. UTD’s current focus will be pregnancy, infectious diseases, drug, dengue, and malaria IVDs.
Apart from serving the Malaysian domestic market, UTD also has orders from other countries to provide OEM and ODM services to meet their needs.
Dato’ Sri Samuel Chai, Chief Executive Officer of UTD shares, “The driver behind this facility is to provide Accurate, Precise and Simple solutions. Covid-19 has shared an experience with us to never take for granted accurate and simple testing at the comfort of our own space”.
As part of the roadmap to improve and to stay ahead of the growing needs in the market and her product offering, the facility is developing it’s own research and development laboratory.
For more information, please contact:
Cassandra Bowden (Miss)
External Communications Executive
Email: cas@universal-therapeutics.com
Tel.: +60 3 9213 0318
About Universal Therapeutics Diagnostics Sdn Bhd
Established in 2021, UTD’s core focus is in the manufacturing and distribution of In-Vitro Diagnostics (IVD). UTD has her own manufacturing facility in Malaysia focused on pregnancy, infectious diseases, drug, dengue, and malaria IVDs. UTD also provides OEM and ODM services. UTD has been certified as a distributor with GDPMD by SGS and as a manufacturer by Medical Device Authority (MDA, Ministry of Health Malaysia).
Universal Therapeutics partners DoctorOnCall for strategic distribution and administration of COVID-19 self-test kits in Malaysia
Kuala Lumpur, Malaysia, 12 October 2021-- Universal Therapeutics, a multinational pharmaceutical and biotech company based in Malaysia, today announced its partnership with DoctorOnCall, Malaysia’s first and largest digital health platform. Consumers can now visit DoctorOnCall’s website and purchase COVID-19 self-test kits at a transparent, fixed price, and have the kits delivered to their homes.
FOR IMMEDIATE RELEASE
Kuala Lumpur, Malaysia, 12 October 2021-- Universal Therapeutics, a multinational pharmaceutical and biotech company based in Malaysia, today announced its partnership with DoctorOnCall, Malaysia’s first and largest digital health platform. Consumers can now visit DoctorOnCall’s website and purchase COVID-19 self-test kits at a transparent, fixed price, and have the kits delivered to their homes.
The partnership addresses the prevalent issue of errant distribution and inconsistent price points of COVID-19 self-test kits in Malaysia.
“Our focus has always been on providing safe and high-quality pharmaceutical and medical products to enhance people’s health and quality of life,” said Sidney Soares, Chief Executive Officer of Universal Therapeutics. “Being able to do so through responsible partners, such as DoctorOnCall, is extremely important to us, so as to ensure that our consumers and patients continue to receive the best care that they need.”
“We resonate with Universal Therapeutics’ mission of providing safe and excellent medical products,” said Hazwan Najib, Co-Founder of DoctorOnCall Malaysia. “In fact, DoctorOnCall was founded to provide quality and affordable healthcare to Malaysians. We reduce waiting time at clinics and hospitals by shifting health consultations online. It is especially critical to provide this service now via a telehealth platform, considering the current pandemic. We are proud to be working with Universal Therapeutics to bring COVID-19 self-test kits to more consumers via our online platform, and look forward to continuing this strong partnership.”
Universal Therapeutics and DoctorOnCall are both committed to aiding the healthcare sector in Malaysia continue their battle against the COVID-19 virus.
###
For more information, please visit https://www.universal-therapeutics.com
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distributes Covid 19 Rapid Antigen tests to wholesalers, medical institutions, and government organisations. All our products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
For more information, please contact:
Samuel CHAI
Group Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com
About DoctorOnCall
DoctorOnCall is Malaysia’s first and leading digital health platform with 2 million Malaysians visiting our website for telehealth consultation, online pharmacy, medication delivery, health content, doctors and specialist booking services. Since its establishment in 2016, DoctorOnCall has established partnerships with top insurers, third-party agencies, hospitals, pharmacies and corporations. These include Prudential, Zurich, Allianz, Great Eastern, Caring Pharmacy, TMC Life Sciences and Columbia Asia Hospitals amongst others.
For more information, visit us at https://www.doctoroncall.com.my/
For more information on the release:
Hazel Hassan
019 217 8191
Medical Device Authority approves TIB SARS-CoV-2 Antigen Rapid Test Kit (Saliva) for Self-Test in Malaysia
Universal Therapeutics, a multinational pharmaceutical and biotech company based in Malaysia, today announced that the Medical Device Authority of Malaysia (MDA) has approved the SARS-CoV-2 Antigen Rapid Test Kit (Saliva) for self-testing.
Manufactured by Triplex International Biosciences (China), the easy-to-use Rapid Test Kit can quickly detect the possibility of a COVID-19 infection, with a sensitivity of 100% specificity of 100% and accuracy of 100%.
FOR IMMEDIATE RELEASE
Kuala Lumpur, Malaysia, 5 October 2021 - Universal Therapeutics, a multinational pharmaceutical and biotech company based in Malaysia, today announced that the Medical Device Authority of Malaysia (MDA) has approved the SARS-CoV-2 Antigen Rapid Test Kit (Saliva) for self-testing.
Manufactured by Triplex International Biosciences (China), the easy-to-use Rapid Test Kit can quickly detect the possibility of a COVID-19 infection, with a sensitivity of 100% specificity of 100% and accuracy of 100%. The test kit works by detecting the presence of the coronavirus nucleocapsid protein in saliva samples. Infants under the age of two are not recommended to use this test kit.
“With the relentless spread of COVID-19, new variants and the huge strain on public healthcare systems, this easy-to-use saliva self-test kit meets the demand for a quick, affordable and accurate way to detect the virus, thereby aiding virus containment efforts,” said Sidney Soares, Chief Executive Officer, Universal Therapeutics.
The kit will be made available through Universal Therapeutics’ network of hospitals, medical facilities, medical institutions and pharmacies. Find out more from your local pharmacy today!
For more information, please visit https://www.universal-therapeutics.com
For MDA’s list of approved COVID-19 test kits, visit https://portal.mda.gov.my/announcement/631-self-test-covid-19-test-kit-for-conditional-approval-approved.html
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distributes Covid 19 Rapid Antigen tests to wholesalers, medical institutions, and government organisations. All our products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
For more information, please contact:
Samuel CHAI
Group Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com
Corporate Misrepresentations
IMPORTANT ADVISORY:
It has come to the attention of Universal Therapeutics that there have been fake brochures, presentations, certificates, and documents ("materials") bearing our company name circulating in forums, social media, and the physical marketplace.
Please ensure the authenticity of the documents by verifying these materials directly with us through legal@universal-therapeutics.com or call our office.
26 July 2021
IMPORTANT ADVISORY:
It has come to the attention of Universal Therapeutics that there have been fake brochures, presentations, certificates, and documents ("materials") bearing our company name circulating in forums, social media, and the physical marketplace.
Please ensure the authenticity of the documents by verifying these materials directly with us through legal@universal-therapeutics.com or call our office.
Universal Therapeutics Sdn Bhd and our associated companies will not take any responsibility for any losses or damages incurred on these such misrepresentations.
Our rights are herein expressively reserved.
Medical Device Authority (MDA)
Medical Device Authority (MDA) Malaysia Approves and Recommends
Hotgen Novel Coronavirus 2019-nCoVAntigen Test Colloidal Gold) Rapid Test Kit.
Universal Therapeutics Sdn. Bhd. is pleased to announce that the Hotgen Novel Coronavirus 2019-nCoVAntigen Test (Colloidal Gold) (“Hotgen Antigen Test”) has been approved and recommended for use by the Medical Device Authority of the Malaysia Ministry of Health (“MDA”).
FOR IMMEDIATE RELEASE
Medical Device Authority (MDA) Malaysia Approves and Recommends
Hotgen Novel Coronavirus 2019-nCoVAntigen Test (Colloidal Gold) Rapid Test Kit
Kuala Lumpur, Malaysia, 01 April 2021
Universal Therapeutics Sdn. Bhd. is pleased to announce that the Hotgen Novel Coronavirus 2019-nCoVAntigen Test (Colloidal Gold) (“Hotgen Antigen Test”) has been approved and recommended for use by the Medical Device Authority of the Malaysia Ministry of Health (“MDA”).
The Hotgen Antigen Test was developed by Beijing Hotgen Biotech Co., Ltd. and boasts a market-leading sensitivity, specificity and accuracy rating of 96.30%, 99.13% and 97.76% respectively. The test detects the presence of viral proteins in acute or early Covid-19 infection by analyzing secretion samples collected using nasal or throat swabs.
“The decision by the MDA to approve and recommend the Hotgen Antigen Test for use in Malaysia paves the way for us to contribute in the fight against the Covid-19 virus and to help Malaysians go about their daily lives safely”, said Chief Executive Officer, Mr. Sidney Soares.
The Hotgen Antigen Test is also approved and used in the United Kingdom- The Medicines and Healthcare products Regulatory Agency and Germany- Federal Ministry of Health (Bundesministerium für Gesundheit, BMG).
Following the positive development on the Hotgen Antigen Test, Universal Therapeutics is working towards the registration of Covid-19 vaccines in Malaysia.
For further information on the Hotgen Antigen Test, you may visit our website at
https://universal-therapeutics.com/ or contact groupsales@universal-therapeutics.com
For MDA’s Approval and Recommendation, visit
https://portal.mda.gov.my/announcement/596-list-of-recommended-for-use-of-covid-19-ivd-test-kit.html
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distribute nitrile gloves; three-ply face masks; KN95 and N95 masks; “HotGen Covid-19 RNA PCR” tests and “HotGen Novel Corona virus 2019-nCoV Antigen (Colloidal Gold) – 15 Minutes Rapid” tests to wholesalers, medical institutions, and government organisations. Their products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
For more information:
Samuel David CHAI
Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com
Bin Suhail International
Bin Suhail International to distribute Covid-19 tests. Abu Dhabi company to market test kits in UAE, Oman, and Saudi Arabia.
Bin Suhail International (Abu Dhabi) has been chosen by Universal Therapeutics Ltd. of Kuala Lumpur as sole distributor in United Arab Emirates, Oman, and Saudi Arabia for its licensed Beijing HotGen Biotech RNA PCR COVID-19 Nucleic Acid, and Beijing HotGen Biotech Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold) test kits.
FOR IMMEDIATE RELEASE
Bin Suhail International to distribute Covid-19 tests
Abu Dhabi company to market test kits in UAE, Oman, and Saudi Arabia
Kuala Lumpur, Malaysia 5 January 2021
Bin Suhail International (Abu Dhabi) has been chosen by Universal Therapeutics Ltd. of Kuala Lumpur as sole distributor in United Arab Emirates, Oman, and Saudi Arabia for its licensed Beijing HotGen Biotech RNA PCR COVID-19 Nucleic Acid, and Beijing HotGen Biotech Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold) test kits.
The appointment also grants Bin Suhail International the rights to distribute other Universal Therapeutics products, including its latex-free disposable Nitrile Butadiene Rubber examination gloves.
“The RNA PCR COVID-19 Nucleic Acid Test Kit is the gold standard for the detection of novel Coronavirus,” said Mr. Sidney Soares, Deputy Chief Executive Officer of Universal Therapeutics. “It detects Covid-19 through a one-step RT-PCR amplification process that identifies the virus in nasopharyngeal swabs, oropharyngeal swabs, and bronchoalveolar lavage fluids, and provides a result in approximately 120 minutes. The test is particularly effective for diagnosing the novel Coronavirus in suspected pneumonia patients, suspected clustering cases, and others requiring differential diagnosis.”
The Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold) is ideal for rural or remote locations. It is easily administered, can be stored at room temperature, is more than 97 percent accurate, and provides results in just 15 minutes. The test detects the presence of viral proteins in acute or early Covid-19 infection by analysing secretion samples collected using nasal or throat swabs.
The powder-free, non-sterile nitrile examination gloves provide a full-textured, enhanced grip with strong puncture resistance. They are perfect for individuals who are allergic to natural rubber latex.
Bin Suhail International will begin accepting orders from customers in United Arab Emirates, Oman, and Saudi Arabia immediately.
To obtain brochures and summary data about the RNA PCR Test COVID-19 Nucleic Acid Test Kit (PCR-Fluorescent Probe Method) and the Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold) rapid test kit, and other products, health care providers are urged to contact: groupsales@universal-therapeutics.com
Contact Details
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distribute nitrile gloves; three-ply face masks; KN95 and N95 masks; “HotGen Covid-19 RNA PCR” tests and “HotGen Novel Coronavirus 2019-nCoV Antigen (Colloidal Gold) – 15 Minutes Rapid” tests to wholesalers, medical institutions, and government organisations. Their products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
Subject to regulatory and safety approval, Universal Therapeutics has also been chosen to distribute Covid-19 vaccinations – in partnership with Beijing HotGen Biotech Co. Ltd. (688068:CH) – by China National Pharmaceutical Group Corporation (Sinopharm).
About Bin Suhail International:
Established in 1982, Bin Suhail International (BSI) is a UAE based local group with a rich diversity in business and delivering exceptional value for our customers and premium returns to stakeholders. We represent international groups in the local market and provide state of art service to ensure that their needs are met. Bin Suhail Group is committed to producing quality goods & services meeting customers specifications and following international standards while complying with local law.
For more information:
Samuel David Chai
Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com
RT-PCR Technique
Universal Therapeutics to offer second Covid-19 test. This test is based on a one-step highly accurate RT-PCR technique.
A second, more comprehensive test for the detection of Covid-19 is available to health care providers in Singapore, Malaysia, Thailand, United Arab Emirates, Kenya, and Ghana. Beijing Hotgen Biotech Co., Ltd. has announced the appointment of Universal Therapeutics as Main Distributor for their RT- PCR COVID-19 Nucleic Acid Test Kit (PCR-Fluorescent Probe Method).
FOR IMMEDIATE RELEASE
Universal Therapeutics to offer second Covid-19 test
This test is based on a one-step highly accurate RT-PCR technique
Kuala Lumpur, Malaysia, 1st December 2020
A second, more comprehensive test for the detection of Covid-19 is available to health care providers in Singapore, Malaysia, Thailand, United Arab Emirates, Kenya, and Ghana.
Beijing Hotgen Biotech Co., Ltd. has announced the appointment of Universal Therapeutics as Main Distributor for their RT- PCR COVID-19 Nucleic Acid Test Kit (PCR-Fluorescent Probe Method).
This is the second Covid-19 test developed by Bejing Hotgen, which also recently selected Universal Therapeutics to be its exclusive distributor for its Novel Coronavirus 2019-nCoV Antigen Test (Colloidal Gold) rapid test kit.
The RNA PCR COVID-19 Nucleic Acid Test Kit is the gold standard for the detection of novel Coronavirus,” said Mr. Sidney Soares, Deputy Chief Executive Officer of Universal Therapeutics. “It detects Covid-19 through a one-step RT-PCR amplification process that identifies the virus in nasopharyngeal swabs, oropharyngeal swabs, and bronchoalveolar lavage fluids. It produces a result in as little as 120 minutes.”
The test is particularly effective for diagnosing the novel Coronavirus in suspected pneumonia patients, suspected clustering cases, and others requiring differential diagnosis.
It is expected that test kits will be available for orders from the date of this news release.
To obtain a brochure and summary data Government bodies, medical institutions and health care providers are urged to contact their offices or visit https://www.Universal-Therapeutics.com for further information.
About Universal Therapeutics:
Universal Therapeutics is a multinational pharmaceutical and biotech company based in Kuala Lumpur, Malaysia. The company’s businesses include manufacturing, distribution and white label production of pharmaceutical products, medical supplies, and PPEs in Asia and throughout the world.
Universal Therapeutics distribute nitrile gloves; three-ply face masks; KN95 and N95 masks; “HotGen Covid-19 RNA PCR” tests and “HotGen Novel Corona virus 2019-nCoV Antigen (Colloidal Gold) – 15 Minutes Rapid” tests to wholesalers, medical institutions, and government organizations. Their products comply with World Health Organization (WHO) guidelines and are accredited and approved by various health ministries as safe for human use.
Subject to regulatory and safety approval, Universal Therapeutics has also been chosen to distribute Covid-19 vaccinations – in partnership with Beijing HotGen Biotech Co. Ltd.
(688068:CH) – by China National Pharmaceutical Group Corporation (Sinopharm).
For more information:
Samuel David Chai
Chief Commercial Officer
Office: +60 3 9213 0318
Email: GroupPR@Universal-Therapeutics.com
Website: www.Universal-Therapeutics.com